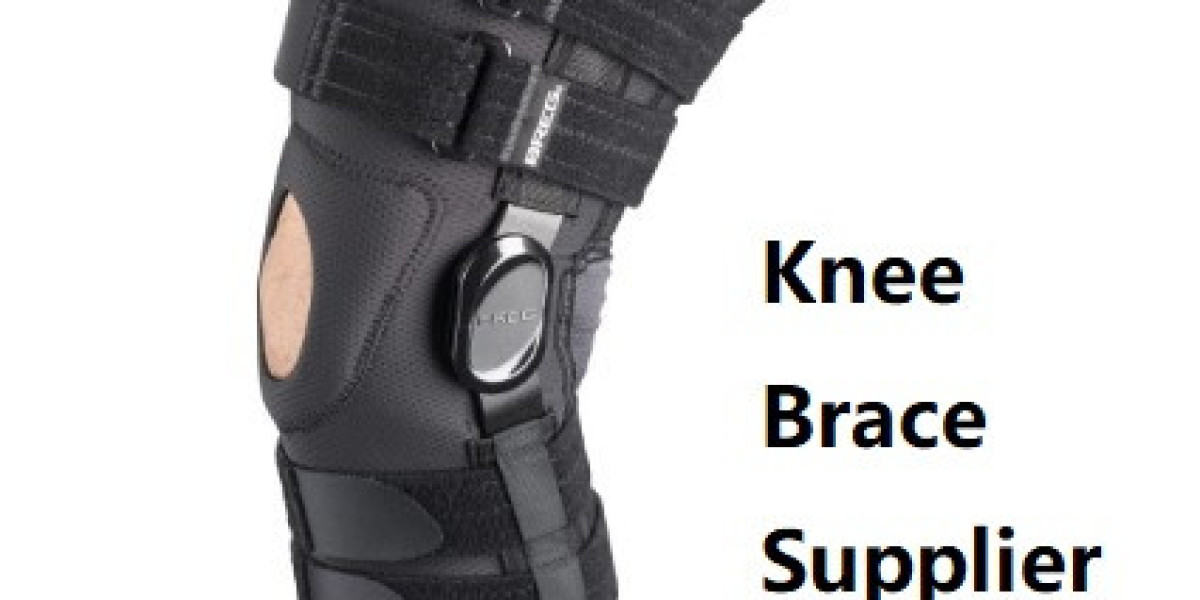
In contemporary healthcare supply chains, clinicians and procurement specialists frequently evaluate options where a Brace Factory provides both standard devices and bespoke solutions, and many prefer a Brace Factory that combines clinical insight with manufacturing excellence. Choosing the right partner affects patient outcomes, clinician satisfaction, and long-term costs — so understanding materials, production workflows, regulatory demands, and design-to-delivery processes is essential. This article outlines how modern brace manufacturers optimize performance, scale production, and deliver patient-centered solutions.
Materials Science and Component Selection
The foundation of any effective orthopedic device is the material system. Engineers select metals, polymers, composites, and textiles based on required stiffness, weight, fatigue resistance, and skin compatibility. Aluminum and titanium alloys offer strength with reduced mass for load-bearing frames, while carbon-fiber composites provide high rigidity for low-weight braces. Thermoplastic polymers enable formable shells that can be thermoformed or injection-molded to precise shapes. For interfaces against the skin, breathable foams and antimicrobial fabrics improve comfort and hygiene. Careful selection and testing of components reduce allergic reactions and improve wear compliance.
Manufacturing Processes and Workflow Efficiency
High-quality brace production blends automated techniques with skilled manual steps. CAD-driven design and CAM machining produce accurate molds and metal parts, while robotic trimming and CNC milling scale repeatability. Thermoforming and injection molding allow rapid production of shells and splints, and ultrasonic welding or adhesive bonding provide secure joins without compromising flexibility. Skilled technicians perform finishing tasks — sanding, padding installation, and strap attachment — to ensure fit and comfort. Streamlined workflows, inventory management, and quality checkpoints minimize lead times and reduce rework, enabling manufacturers to meet urgent clinical schedules.
Steriger Innovations in Customization
Steriger Customization Capabilities
Personalization is increasingly critical in orthopedics. Digital scanning — using handheld scanners or CT-derived models — feeds into CAD systems to generate anatomically precise braces. Additive manufacturing (3D printing) produces complex lattice structures for targeted flexibility and ventilation. Modular component systems let clinicians configure hinge placement, range-of-motion stops, and adjustable supports without full remanufacture. This combination of digital design and modular hardware allows rapid prototyping, fast clinical iterations, and patient-specific tuning that improves therapeutic outcomes.
Regulatory Compliance and Quality Assurance
Medical devices must meet stringent regulatory standards. Manufacturers implement ISO 13485-compliant quality management systems to ensure traceability, consistent processes, and controlled documentation. Biocompatibility testing, fatigue and load testing, and flammability assessments are routine for materials and finished devices. Clear labelling, sterilization protocols (where applicable), and packaging validation protect devices through shipping and handling. Post-market surveillance and complaint handling close the loop on design improvements. Rigorous compliance reduces liability and increases clinician trust.
Clinical Integration and Training
A successful brace is more than hardware — it’s part of a care pathway. Manufacturers provide clinician training on fitting techniques, adjustment protocols, and outcome measurement. Instructional videos, fitting guides, and clinical decision aids support adoption in busy practices. Some factories partner with rehabilitation teams to collect wear-time and patient-reported outcome data, informing iterative design changes. By supporting training and integration, manufacturers help clinicians maximize therapeutic benefits for patients while reducing improper use and returns.
Sustainability, Supply Chain Resilience, and Future Trends
Sustainable practices are shaping modern production. Recyclable polymers, reduced solvent usage, and energy-efficient manufacturing lower environmental impact. Standardized modules and repairable components extend product life, and take-back or refurbishment programs reduce waste while cutting costs. Supply chain resilience — multiple sourcing for critical components and localized production hubs — protects delivery schedules during disruptions. Future innovations include smart braces with embedded sensors for gait analysis, materials that change stiffness on demand, and broader use of AI in design optimization. These advancements promise braces that are lighter, smarter, and more effective.
Selecting the right brace manufacturer matters for clinical efficacy, patient comfort, and operational efficiency. By combining rigorous materials selection, modern manufacturing workflows, regulatory compliance, clinician support, and forward-looking sustainability, leading factories help healthcare teams deliver better care. For further information about Steriger products, technical resources, and contact details, visit www.steriger.com/